Study title: Is the naturally occurring prebiotic Lactoferrin an acceptable alternative to antibiotic/antifungal tablets for women with bacterial vaginosis or thrush? The LISA (
Lactoferrin
In
Stead of
Antibiotics/antifungals) randomised feasibility study.
Bacterial vaginosis (BV) and vaginal thrush/candida are common infections affecting over 200,000 women each year in England, with an estimated annual cost to the NHS of more than £60 million.
Symptoms of BV and candida may include abnormal vaginal discharge and soreness, itching or burning in and around the vagina. BV can also cause an unpleasant fishy smell. Women with BV are at increased risk of HIV infection, pelvic inflammatory disease, late miscarriage and preterm birth. Similarly, vaginal thrush may be associated with HIV infection and preterm birth which is the main cause of neonatal death and disability worldwide.
In the UK an estimated 1.2 million women have recurrent vaginal infections. Although some women report little impact on their lives, in others recurrent infections can be socially and sexually disabling, affecting their relationships, self-esteem and quality of life.
Both BV and vaginal thrush are usually treated with oral antibiotics/antifungals. Unfortunately, about a third of women suffer recurrent infections. These women may be given multiple courses of antibiotics which may cause side effects and increase the risk of antimicrobial resistance.
We want to see if naturally occurring Lactoferrin (made from cows’ milk) might be an alternative to antibiotic/antifungal tablets for women with bacterial vaginosis or thrush.
What is lactoferrin?
Vaginal lactoferrin is made from cows’ milk. It encourages the growth of healthy lactobacilli in the vagina and may prevent bacterial vaginosis and thrush.
Lactoferrin has been used in research studies by nearly 200 women (including during pregnancy) with no recorded adverse events.
In 2019-2020, women bought over 60,000 boxes of vaginal lactoferrin (Difesan) and no serious adverse events were reported.
LISA is an open label randomised feasibility study to investigate if a future definitive trial can be conducted which proves that vaginal lactoferrin is an acceptable, safe and effective alternative to antibiotics/antifungals for women with Bacterial vaginosis or thrush.
Sponsors and funding
The study is funded by the National Institute for Health and Care Research under its Research for Patient Benefit programme (RfPB) and is sponsored by City St George's, University of London.
Trial design
LISA is an open label randomised feasibility trial over 12 weeks with qualitative, microbiological, and economic evaluations.
Trial setting
For this feasibility study we will only recruit women attending the Burrell Street Sexual Health Clinic or the Curran General Practicein London.
Target population
A total of 114 women will be recruited for this study.
Who can take part
Women who:
- are aged 16 to 49 years who have vaginal symptoms due to bacterial vaginosis or thrush
- have periods (apart from women with a Mirena IUCD or polycystic ovary syndrome)
- have a clinical diagnosis of bacterial vaginosis or thrush confirmed on Gram stain.
Trial flow chart
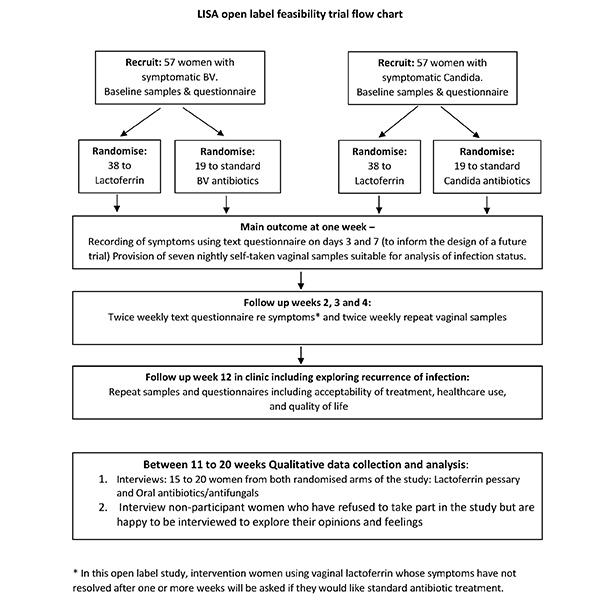
View the trial flow chart document here (Word).
Trial treatments
Intervention arm: Vaginal lactoferrin
38 women with bacterial vaginosis and 38 women with Candida will be given bovine lactoferrin 300mg vaginal pessaries (Difesan, Uriach, Italy) to insert every evening for the first 21 days of the study.
Control arm: Usual oral antibiotic/antifungal treatment
19 women with bacterial vaginosis will be given metronidazole 400mg twice daily for 5 days. 19 women with candida will be given a fluconazole 150mg capsule to take the same day.
Outcomes
- Acceptability of vaginal lactoferrin from questionnaires and interviews
- Adherence to treatment (from participants’ weekly count of remaining pessaries/tablets)
- Recruitment rate and willingness of women to be randomised
- Follow-up rate
- Acceptability of study procedures such as providing vaginal samples and responding to texts about symptoms
- Adverse events
- Estimate of the cost of lactoferrin and the feasibility of obtaining data on healthcare use.
- Percent of participants completing symptom questionnaires
- Percent of participants providing samples suitable for microbiological analyses (e.g. To assess for a healthy lactobacilli-dominated vaginal microbiome) to inform the design of a future definitive trial.
What happens to women who take part in the study?
Week 1: Provide self-taken vaginal samples at home every evening for 7 days. Receive text questionnaires on day 4 and 7.
Week 2, 3 and 4: Vaginal samples taken at home in the evening twice a week. Receive text twice a week to ask about symptoms.
Week 5 to 11: No contact.
Week 12: Invited back to clinic or general practice for a final check up.
Trajectory to patient benefit
If this study leads to a future trial which proves that vaginal lactoferrin is an acceptable, safe, and effective alternative to antibiotics, using lactoferrin could:
- help relieve symptoms
- reduce recurrence rates
- prevent antimicrobial resistance
- save NHS costs.
Future research might show if lactoferrin could also reduce infection-related preterm birth.
Payments
Participants will be given a £30 honorarium when they attend for the final 12-week check. Those who take part in interviews will receive an additional £10.