This article is part of a series of research impact stories related to our REF 2014 submission.
City St George’s Professor Mike Sharland is developing new tools and treatments to fight infections in children and halt the spread of antibiotic resistance.
The emergence and spread of drug-resistant bacteria is an emerging public health crisis. The antibiotics that we rely on to combat bacterial infections are rapidly becoming ineffective, causing thousands of people to die from conditions that should be easily treatable.
33,000 people in Europe die each year from antibiotic-resistant infections
Despite high infection rates, antimicrobial resistance in children is poorly researched. ‘Studies are usually done in adults first and then there is often a long delay before any studies are done in children, or indeed they never done at all,’ says Professor Mike Sharland, who was recently awarded a CBE for his work on antimicrobial resistance.
Sharland points out that the World Health Organisation’s antibiotic treatment regimens have been the same for over 50 years. ‘Most kids have infections that are resistant to these drugs,’ says Sharland. ‘But new antibiotics are completely unaffordable for most of the children in the world.’
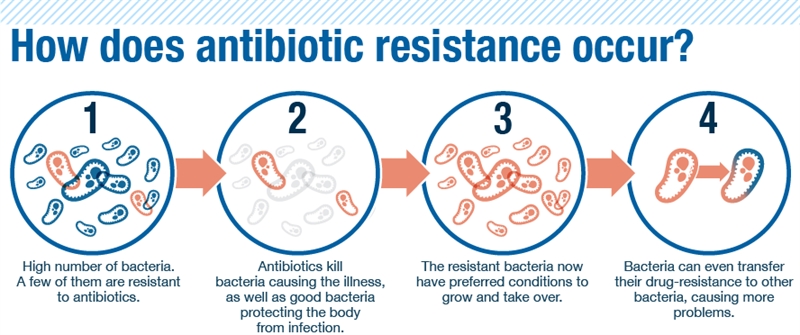 The process for bacteria becoming resistance to antibiotics.
The process for bacteria becoming resistance to antibiotics.
Instead, Sharland and his colleagues are investigating how best to use these older, existing drugs to treat infections in children while limiting side effects and preventing the spread of antimicrobial resistance.
‘Our work has helped to inform the WHO to develop new metrics, new ambitions and new targets,’ says Sharland, who helped to develop a system known as the AWaRe index. The index divides antibiotics into three groups to help doctors select the best medicines to treat children and prevent resistance:
-
‘Access’ antibiotics are the first choice for common infections
-
’Watch’ covers a broader range of antibiotics which should be used sparingly
-
’Reserve’ antibiotics should only be used as a last resort for treating multidrug-resistant infections.
In a recent study funded by the PENTA Foundation, Sharland and his team used the AWaRe index to investigate antibiotic prescribing in children around the world.
Based on this work, the WHO announced their first hard target designed to tackle antimicrobial resistance in children, which stipulates that 60% of prescriptions should be for access antibiotics.
‘Too many infections are being treated with broad-spectrum antibiotics,’ Sharland says. ‘It is most important that we encourage improved access to Access antibiotics to treat infections in children globally if we are to tackle the problem of antibiotic resistance.’